M. Badawi and S. Gopalakrishnan equally contributed to this work
Introduction: Venetoclax is a selective BCL2 inhibitor approved for patients with CLL and patients with newly diagnosed AML who are ineligible for induction chemotherapy. Venetoclax is currently being evaluated in other hematologic and solid tumors in both adult and pediatric populations. In this analysis, we characterized the pharmacokinetics and exposure response relationships of venetoclax to guide dose selection of venetoclax in combination with high dose cytarabine (HDAC) in pediatric patients with relapsed or refractory AML.
Methods: A population PK model describing venetoclax PK in pediatric patients was developed using data from 121 subjects across three venetoclax studies that tested venetoclax at 400-800 mg doses across different tumors. The model accounted for the CYP3A4 developmental changes in early years of life using a maturation function and allometric scaling was also incorporated in the model to account for growth and body size effect. Individual subject average venetoclax plasma concentration (Cavg) up to the event of interest, determined based on the population PK analysis using the post-hoc empirical Bayesian parameter estimates, was used as an exposure metric to explore exposure-efficacy and exposure-safety relationships. Data from subjects with AML (database version 23-Mar-2020) who received venetoclax in combination with either azacitidine, decitabine, or cytarabine were included in the exposure-efficacy and exposure-safety analyses. The population PK model was then used to simulate venetoclax exposures in pediatric subgroups to guide dose selection for future trials.
Results: Population PK estimates (clearance, volume of distribution, rate of absorption and peripheral volume of distribution) were comparable to previously reported estimates. Additionally, weight was identified as a statistically significant covariate on clearance and volume of distribution.
Exposure-response analyses showed a flat relationship between venetoclax exposures and efficacy in patients with AML who received venetoclax in combination with azacitidine, decitabine, or cytarabine. Moreover, venetoclax exposures did not correlate with incidence of neutropenia. Specifically, in AML patients who received venetoclax in combination with HDAC (>500-2000 mg/m2/day, n=15), higher venetoclax exposures were not associated with better clinical response. A 600 mg dose of venetoclax was selected based on high response rates observed in the clinic.
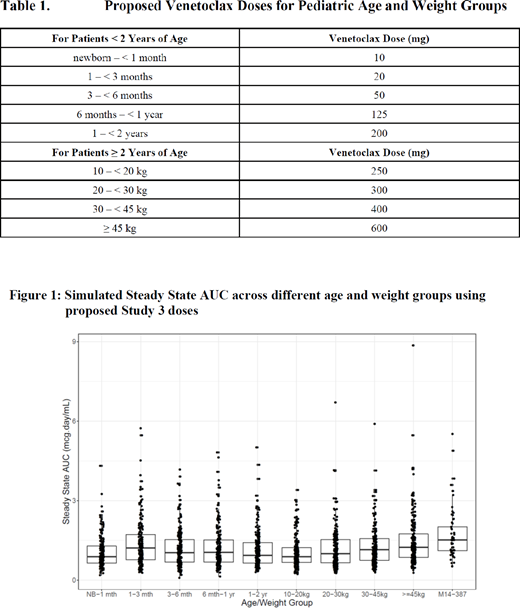
Venetoclax doses for pediatric subgroups were selected based on weight (allometric scaling) for children aged ≥ 2 years old and based on weight and CYP3A ontogeny for children aged < 2 years old. Table 1 presents the selected doses for the pediatric age and weight groups. Simulations of exposures (steady state area under the curve [AUCss]) using the population PK model for the pediatric age and weight groups are represented in Figure 1. Reasonable overlap in exposures was observed across the pediatric groups.
Conclusions: Exposure-efficacy and exposure-safety showed lack of superiority of higher exposures and hence, 600 mg QD venetoclax in combination with HDAC was selected for pediatric patients with relapsed or refractory AML in future trials. Simulations of exposures demonstrated that the selected age- and weight-based doses would achieve exposures in pediatric subgroups comparable to that expected in adults receiving a 600 mg dose of venetoclax.
Badawi:AbbVie Inc.: Current Employment, Other: may hold stock or other options. Gopalakrishnan:AbbVie Inc.: Current Employment, Other: may hold stock or other options. Engelhardt:AbbVie Inc.: Current Employment, Other: may hold stock or other options. Palenski:AbbVie: Current Employment, Other: may hold stock or other options. Kim:AbbVie, Inc.: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Other: may hold stock or other options. Karol:AbbVie Inc.: Other: Unrelated to this study, St. Jude has received a charitable contribution from AbbVie, Inc. The charitable contribution is not being used for clinical or research activities, including any activities related to this study. . Rubnitz:AbbVie Inc.: Research Funding. Menon:AbbVie Inc.: Current Employment, Other: may hold stock or other options. Salem:AbbVie Inc.: Current Employment, Other: may hold stock or other options.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal